Mein Leitfaden für eine perfekte VMK-Krone: Metalloxidation und Haftungsversagen stoppen
I’ve been working with dental materials for a long time. I have seen beautiful work that lasts for decades. I have also seen crowns that fail way too soon. The difference often comes down to one thing: the bond between the porcelain and the metal. A weak bond can lead to chips, cracks, and a complete failure of the crown. This is a big headache for the dentist, the lab, and most of all, the patient.
In this article, I am going to pull back the curtain. I will share my experiences with the PFM metal oxidation process. I will show you why it is so important. You will learn the secrets to creating a strong, lasting porcelain-metal bond. By the end, you will know how to spot problems before they start. You will learn how to prevent the costly and frustrating issue of bond failure.
Inhaltsübersicht
Was ist eine PFM-Krone überhaupt?
Let’s start with the basics. PFM stands for porcelain-fused-to-metal. Think of it as a tooth made of two layers. The inside layer is a strong metal cap that fits over the tooth. We call this the metal framework or substructure. The outside layer is porcelain, which is a type of ceramic. The porcelain is shaped and colored to look just like a natural tooth. The PFM-Krone has been a workhorse in dentistry for over 50 years.
The magic of a PFM crown is that it combines strength and beauty. The metal inside gives it the power to stand up to chewing. The porcelain outside makes it look good in the patient’s smile. But this only works if the two layers are stuck together perfectly. The process of making that bond is where many labs go wrong. I have seen countless cases where a simple mistake in the lab leads to a big problem later.
Warum ist der Metalloxidationsschritt so wichtig für eine gute Verbindung?
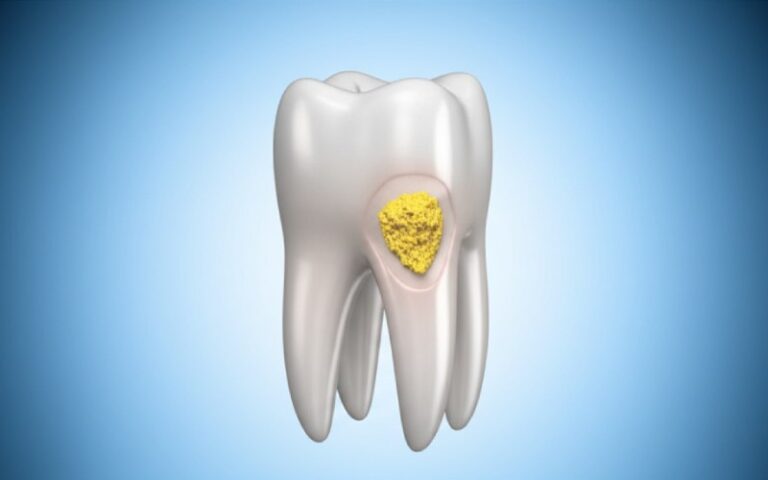
When you hear the word “oxidation,” you might think of rust on an old car. In the case of a PFM crown, oxidation is not a bad thing. It is a very good and very necessary thing. We are not talking about rust. We are talking about creating a special, very thin layer on the metal. This is the oxide layer. We create this layer by heating the metal framework in a special oven before we add any porcelain.
This oxide layer acts like a primer. Imagine you are going to paint a shiny metal pole. If you just put paint on the smooth metal, it will peel right off. But if you put a special primer on first, the paint will stick like glue. The oxide layer is the primer for the porcelain. It creates a chemical bridge between the metal and the porcelain. Without the right oxide layer, you will never get a strong and reliable chemical bond.
How Does the Porcelain Stick to the Metal Frame?
The bond between the porcelain and the metal is not just one thing. It is actually a team of three things working together. I like to think of them as three legs on a stool. If one leg is weak, the stool will wobble and fall.
First, you have the chemical bond. This is the strongest and most important part. The special oxide layer we just talked about melts and mixes with the first layer of porcelain. This first layer is called the opaque porcelain. It creates a true chemical connection. This is the main source of the bond’s amazing tensile strength.
Second, you have mechanical retention. Before we heat the metal, we make its surface a little rough. We do this with a process called sandblasting. The rough surface has tiny hills and valleys. The liquid porcelain flows into these tiny spaces. When it cools and hardens, it is locked into place. This adds a lot of strength.
Third, you have compressive forces. The metal and the porcelain are designed to shrink a little as they cool. The porcelain is designed to shrink just a tiny bit more than the metal. This slight difference in thermal expansion puts the porcelain under compression. It’s like the porcelain is being gently squeezed onto the metal. This makes it very strong and resistant to chipping.

What Are the Main Causes of a Porcelain-Metal Bond Failure?
I’ve seen bonds fail for many reasons. But most of the time, it comes down to a few common mistakes. The number one enemy of a good bond is contamination. Even a tiny bit of oil from your fingers can ruin the chemical bond. It stops the oxide layer from forming correctly.
Another big problem is an improper degassing cycle. This is when you heat the metal to burn off any impurities and to form that perfect oxide layer. If you don’t heat it hot enough or long enough, you get a weak oxide layer. If you heat it too hot, the metal can warp.
Sometimes, the problem is with the casting of the metal itself. If the metal has bubbles or is not dense enough, it creates weak spots. The bond will fail in those areas. Finally, using the wrong type of metal or porcelain can cause problems. The thermal expansion of both materials must match very closely. If they don’t, the bond will be under constant stress. It’s a ticking time bomb waiting to fail.
Are Noble Alloys Better Than Base Metal Alloys for PFM?
This is a question I get all the time. There are two main families of metals we use for PFM crowns. We have noble alloys and base metal alloys. Noble alloys contain a high amount of precious metals, like gold or palladium. Base metal alloys are made of metals like nickel, chromium, or cobalt.
In my experience, noble alloys are often easier to work with. They form a very predictable and reliable oxide layer. This makes it easier to get a strong chemical bond. They are also very kind to the gums. The downside is that they are much more expensive.
Base metal alloys are very strong and much cheaper. They are a great choice when cost is a concern. However, they can be trickier to work with. Creating the perfect oxide layer on a base metal alloy takes more skill and care. If you don’t do it right, you can get a dark, ugly oxide that shows through the porcelain. But when handled by a skilled technician, a base metal PFM can be just as good as one made from a noble alloy.
Can a Dirty Surface Really Cause the Bond to Fail?
Yes, absolutely! I cannot say this enough. Surface contamination is the silent killer of PFM crowns. After the metal framework design is finished and the casting is done, it must be handled with extreme care. The technician grinds and shapes the metal. This creates dust and debris.
Before the oxidation or degassing step, the metal must be perfectly clean. We use steam cleaners or special solutions to wash away every trace of dirt. Even a fingerprint can leave behind oils that will block the chemical bond. The oils burn away in the oven, leaving a spot where the oxide layer cannot form. The porcelain in that spot will have nothing to stick to. This creates a weak point that can lead to a chip or a complete bond failure years later.
What Is Degassing and Why Must I Do It Every Time?
Degassing is the name for the first heating cycle we do on the metal framework. We do it before we add any porcelain. This step has two very important jobs. First, it burns away any hidden impurities or surface contamination that might be trapped in the metal from the casting process. This ensures the surface is pure.
Second, it grows the oxide layer. The heat and the air in the oven work together to form that perfect, thin layer of metal oxides. This is not something you can see easily. It is a microscopic layer. Skipping the degassing step is like building a house with no foundation. The first layer of opaque porcelain will have nothing to bond to. The entire Veneers porcelain could pop off later. In my lab, every single PFM framework goes through a proper degassing cycle. No exceptions.
How Does Sandblasting Help Create a Stronger Bond?
Remember when I talked about mechanical retention? This is where sandblasting comes in. Sandblasting is a process where we shoot very tiny, hard particles at the metal surface. We use a material called aluminum oxide. It is like sand, but much harder and sharper.
This process does not damage the metal. It just makes the surface rough on a microscopic level. It creates millions of tiny nooks and crannies. This rough surface gives the porcelain something to physically grab onto. When we apply the first layer of opaque porcelain, it flows into all these tiny spaces. When it is fired in the oven, it hardens and becomes locked in place. This physical lock works together with the chemical bond to create a bond that is incredibly strong.
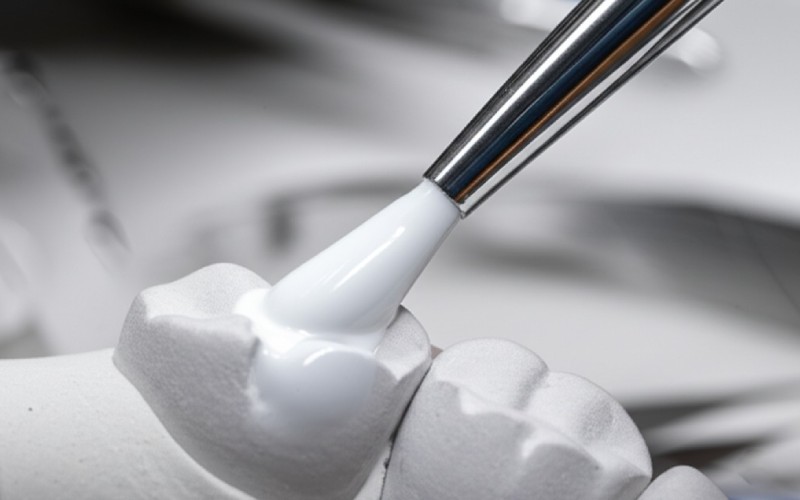
Does the Metal Framework Design Affect the Bond?
The shape of the metal framework is very important. A good framework design supports the porcelain and protects it from breaking. The metal should not be too thick or too thin. If it’s too thin, it can bend when the patient chews. This bending puts a lot of stress on the porcelain and can cause the bond to break or the porcelain to crack.
A good design also avoids sharp angles. Sharp inside corners on the metal create stress points. The porcelain is much more likely to crack in these areas. All the angles on the framework should be smooth and rounded. The design must also make sure the layer of veneer porcelain is an even thickness. If one area has very thick porcelain, it is more likely to break. A good lab technician knows that the design is the first step to a long-lasting crown.
What Are the Signs of a Weak Bond I Can See?
Sometimes, you can see the signs of a weak bond before the crown even leaves the lab. One of the biggest red flags is a dark line at the edge of the crown. This can mean the oxide layer was too thick and is showing through the opaque porcelain. This thick, dark oxide often creates a very weak bond.
Another sign is when the opaque layer looks uneven or has bubbles in it. This could be a sign of surface contamination on the metal before the porcelain was added. If you ever see a finished crown where the porcelain looks dull or does not reflect light properly, it can be a sign of a problem with the bond underneath. As a dentist, if a PFM crown doesn’t look quite right, it is always better to ask questions. A small issue in the lab can become a big failure in the mouth.
Summary: My Keys to a Perfect PFM Bond
I’ve shared a lot of information from my years of experience. If you remember nothing else, remember these key points. They are the foundation of a successful PFM crown and will help you avoid the dreaded porcelain-metal bond failure.
- Start with a Clean Surface: Always make sure the metal framework is perfectly clean before every step. Surface contamination is the number one enemy.
- Don’t Skip Degassing: The degassing cycle is critical. It cleans the metal and creates the all-important oxide layer needed for a strong chemical bond.
- Get the Oxide Layer Right: The oxide layer must be thin and even. Too thick or too thin, and the bond will be weak. This is controlled by the oven temperature and time.
- Use Mechanical Retention: Proper sandblasting creates a rough surface that gives the porcelain something to hold onto. It’s a key part of the team.
- Design for Success: A good framework design supports the porcelain and avoids sharp angles that create stress points.
- Match Your Materials: Make sure the thermal expansion of your metal alloy and your veneer porcelain are a good match to prevent stress during cooling.